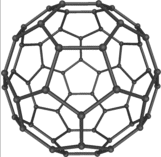
 There's an interesting science program on Radio 4 called The Material World that I often catch when I'm in the car driving from work to pick Nikki up. Today it was about something called Graphene, which I'd never heard of before but which sounds very exciting. I knew of Buckminsterfullerene (or bucky balls) which is made of a number of carbon atoms - 60 if I remember right - arranged in a sphere or ball shape and is one of the most stable carbon molecules to be discovered. It's named after Buckminster Fuller, the American architect who invented the geodesic dome, because the carbon atoms are laid out in hexagons and pentagons around the face of the sphere in exactly the same way as a dome is constructed.
There's an interesting science program on Radio 4 called The Material World that I often catch when I'm in the car driving from work to pick Nikki up. Today it was about something called Graphene, which I'd never heard of before but which sounds very exciting. I knew of Buckminsterfullerene (or bucky balls) which is made of a number of carbon atoms - 60 if I remember right - arranged in a sphere or ball shape and is one of the most stable carbon molecules to be discovered. It's named after Buckminster Fuller, the American architect who invented the geodesic dome, because the carbon atoms are laid out in hexagons and pentagons around the face of the sphere in exactly the same way as a dome is constructed.Well graphene, it turns out, is a planar sheet of carbon atoms just one atom thick - a bit like a bucky ball that's been unzipped and laid out flat. Actually that's not entirely accurate because to be a perfectly flat sheet the atoms must be laid out in hexagonal cells only. The pentagons that are necessary to achieve a spherical shape, if present in graphene, cause it to bend into other shapes. Carbon nanotubes, which have caused such excitement recently in the field of nanotechnology because of their potential use in building very small structures, are simply rolls (cylinders) of graphene.
The really exciting thing about this stuff is that electrons on a sheet of graphene act as if they have no mass. They move as if they were light waves. This makes graphene extremely conductive (I think the guy on the radio show said something like a thousand million times more conductive than copper) and hence a much better bet to use in microchips. Since its isolation in 2004 graphene has become relatively easy and cheap to manufacture (it looks like soot) and physics is now in the middle of an experimental phase learning more about its amazing properties before coming up with real-world applications. The potential for extreme microminiaturisation using graphene is high, and graphene-based transistors have already been made.
If you're interested there's more on Wikipedia (as always!) and some of the external links at the bottom of that page make fascinating reading too.




No comments:
Post a Comment